Instead of a few months, it took them 2 years. But it’s also a world premiere: Perfood has developed the first digital therapy against migraine based on nutritional medicine that was approved by a medical regulatory body. CEO Dominik Burziwoda shares the journey and his learnings in this interview.

Founder and CEO, Perfood
Dominik Burziwoda is the founder and CEO of Perfood, a Germany-based digital therapeutics startup and portfolio company of Verve Ventures. Perfood has developed proprietary technology for personalized low-glycemic diets as the core mode of action of its digital therapeutics products. The company already launched a consumer product called MillionFriends in 2018. It is aimed at self-payers interested in improving their general health through optimal nutrition.
Prior to founding Perfood, Dominik was responsible for the operations of an international e-commerce startup after a career in investment banking and corporate finance. He graduated with a Master’s in Finance from IE Business School, a Master’s in Law (LL.M.) from the University of Münster and a BSc in International Business Administration from Rotterdam School of Management, Erasmus University.
In October 2022, Perfood’s migraine therapy called sinCephalea (from Latin: without headache) received approval from the German regulator (the German Federal Institute of Drugs and Medical Devices, BfArM) as digital therapy under the Digital Care Act (DiGA). This means that the product is covered by all German statutory health insurance groups without any surcharge for patients. These insurance groups represent approximately 70 million German citizens. In Germany alone, around 7-10 million patients suffer from migraines, headaches that can cause severe pain.
How does your digital therapy alleviate migraines?
Our brains consume a lot of energy – around 20% of what our bodies need to function. The ability to concentrate and focus comes from having plenty of energy, which is provided in the form of glucose in our blood to the brain. If glucose levels drop, we get “hangry”, which means we’re irritable or angry because of hunger. Eating sugary meals may cause headaches by driving a blood sugar spike that’s often followed by a crash. This cycle of glycemic variability may trigger both regular, tension-type headaches and migraines. The challenge, however, is that each person responds completely individually to such meals. Hence, a personalized approach is the only road to success when treating migraines with a “low-glycemic diet”.
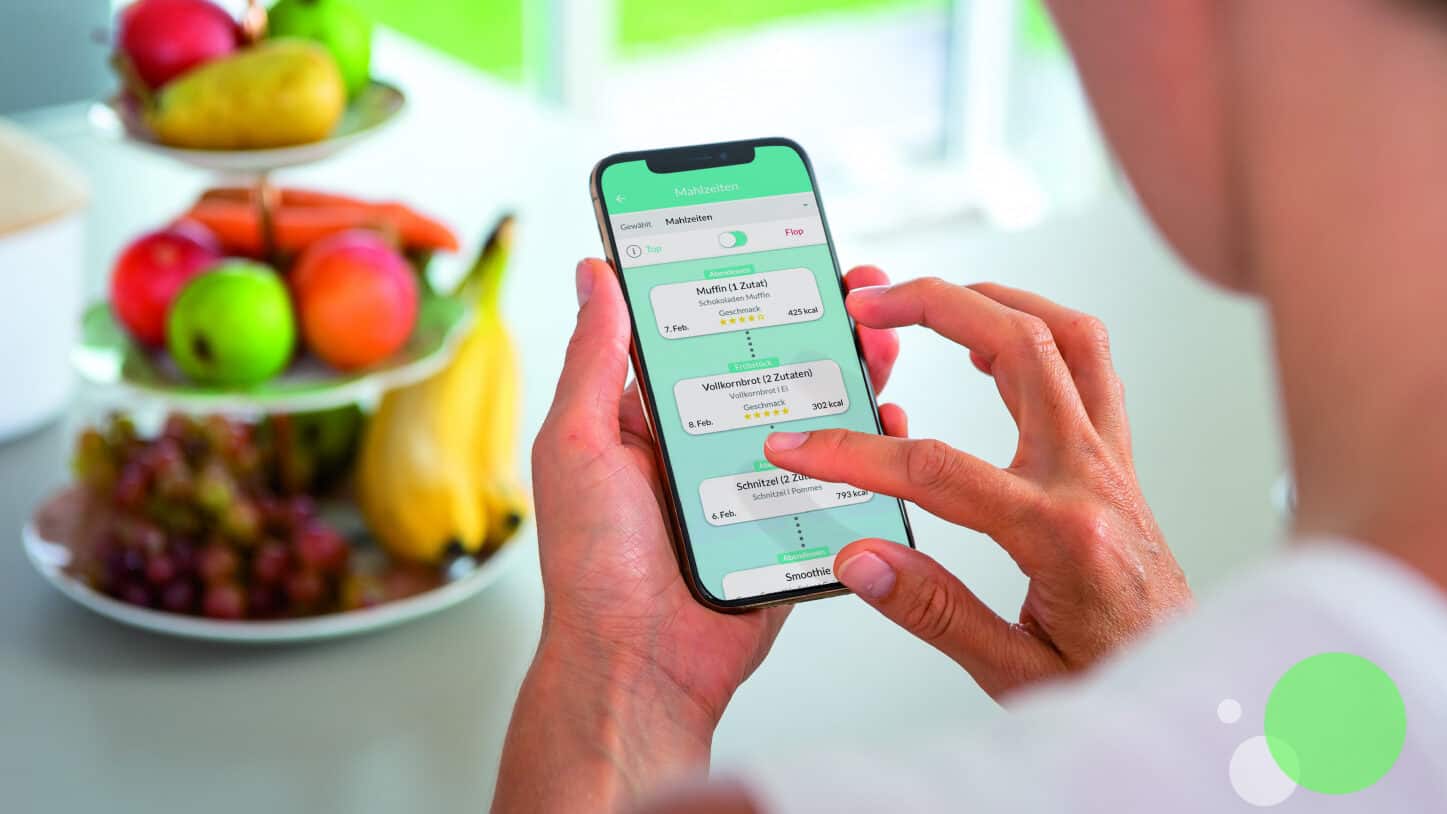
sinCephalea is an app that works with continuous glucose monitoring sensors of all major manufacturers. In an initial test phase of up to 2 weeks, patients take notes about the meals they eat in the app while the sensors measure the individual reaction of the patients to the different types of food in terms of blood sugar spikes. It then establishes a list of foods that lead to stable glucose levels (good) and those that lead to spikes in glucose levels (bad). Adapting one’s eating habits towards foods that release glucose slowly into the bloodstream helps keep people mentally alert throughout the day and significantly reduces the length of migraine and headache episodes and their frequency. This means that patients need to take fewer medications such as headache pills and triptans which are used to treat symptoms of migraines, but are not a cure as well as beta blockers which reduce blood pressure for migraine prophylaxis.
“You can improvise a lot of things as a startup, but not the documentation you need to hand to the regulator.”
What are your key takeaways from the process?
As a manufacturer, especially as a young company, you need to be aware of the fact that clearing the regulatory hurdles requires a very high degree of professionalism. You can improvise a lot of things as a startup, but not the documentation you need to hand to the regulator. They are used to dossiers from big pharma companies that are top-notch. They will try to figure out where the weaknesses in your application are – this is their job. We received a lot of queries, over and over again, and every time we had to say: well, they have a point here. The second important takeaway is that it will take longer and cost a lot more money than you expect. This is bad news for all startups that aren’t very solidly financed. The whole process has cost us more than 50x of what we originally expected if you consider that it took two years.
What does the regulatory approval for sinCephalea mean for Perfood?
There are several aspects to it. First, it allows us to significantly increase our sales. We achieve margins that allow us to run a sustainable business. If we want, we could be break-even next year. This flexibility is important to investors who have become more selective. Second, it improves our reputation as a company. It shows that we are able to bring a digital therapy through the regulatory process. We’ve learned a lot during the two years that this took us. This makes Perfood more attractive for big corporate partners such as pharma or tech firms that think about launching digital therapies. Third, it has greatly improved the spirit in our team. We were lucky to have supportive and patient investors, and we were busy improving our product and selling MillionFriends, but this is a major breakthrough that has lifted all spirits.
How did you celebrate this milestone as a team?
The days leading up to the decision were quite nerve-racking. The deadline for the submission fell on a Thursday. On Monday, I phoned the regulator to find out where we stand. There wasn’t a decision yet, but I was told it looked good. Only then did we start preparing the material for market entry such as the product website and the press release – we didn’t want to spend money without knowing we would get the approval. It took two more weeks until we got the green light on a Friday, and I posted in our chat that today is a pretty good day – we’re finally DiGA. Since we work remotely, groups of our team gathered in different cities, Hamburg, Lübeck and Berlin to celebrate with each other.
The DiGA regulatory regime was only introduced in 2020. It is widely seen as a pioneering framework for digital therapies and has brought Germany to the forefront of innovation in this space. As Perfood’s experience shows, it’s also quite a high hurdle. How would you explain DiGA to someone who is not familiar with it?
To understand the goal of regulation in the field of pharmaceuticals you need to understand that the German regulator BfArM was established after the Contergan scandal that was uncovered in the early 1960s. More than 10’000 children worldwide were born with deformities and many of them subsequently died because their mothers took the tranquilizer Contergan while they were pregnant. Strict regulation was established with the aim of never letting such a medical disaster ever happen again. So you can say that BfArm was established with a risk-reduction goal in mind.
Now, with the establishment of DiGA, the legislator wanted to promote innovation and digitalization in the field of medical care. It can be seen as an economic policy to create a favorable environment for innovation. With regard to DiGA, BfArM now also does the reimbursement-access assessment, which means they decide which therapies get reimbursed by health insurers. But since they come from this risk-assessment perspective, they have a very high bar for this decision. They have been criticized by founders who would like the regulator to be more accommodating. On the other hand, taxpayers’ money should not be squandered to pay for therapies with limited evidence. But one can argue that such therapies would also not be very likely prescribed by doctors. In the end, the regulators’ decision to approve a therapy carries a lot of weight, not just for German patients. Many countries are looking very closely at what is happening in Germany right now.

Invest in Startups
As one of Europe’s most active venture capital investors, we grant qualified private investors access to top-tier European startups. With investments starting at EUR/CHF 10’000, you can build your own tailored portfolio over time and diversify across stages and sectors.
So digital health startups need millions to develop what many people still see as mere gimmicks, not real medicine. Why would investors care?
Because they understand that as a society, we have an enormous problem to solve. Heart attacks, strokes, diabetes, and certain forms of cancer, the biggest burdens on our healthcare systems all have their root causes in unhealthy lifestyles. In Germany, over 14% of our salaries go towards healthcare, a figure that will rise to 22% by 2030. Baby boomers have just started retiring. Our healthcare systems devote around 90% of their spending to curing the effects of lifestyle-associated diseases. Digital therapies are our best shot at reigning in these spiraling costs. This field is just getting started, digital therapies will become better and cheaper. And they don’t have any adverse side effects. Do you really want to prescribe a 20-year-old patient beta blockers with all their adverse side effects for the next 3 decades to alleviate migraine or would you prefer an effective therapy without adverse side effects? The answer should be clear, also from a cost perspective. But yes, there is a lot of education still to be done. We still need to do a lot of studies to convince people that digital therapies are serious medicine.
Speaking of which, your big clinical trial for migraine is currently still ongoing. How could your product get approval while this trial hasn’t finished yet?
We have received preliminary approval for 10 months, which can be applied for using pilot data. All in all, we did 3 preliminary studies. Our first study was rudimentary, we filed the first application with it but didn’t receive approval. The second was more comprehensive, and we published the results in the Journal of Clinical Medicine. But it was a retrospective study, where we asked patients after the treatment, which again wasn’t good enough for the regulator. So we did two prospective studies, published the results in the peer-reviewed publication Nutrients, and those are the ones the current regulatory approval is based on. To confirm the findings, we’re now running the clinical study you mentioned, which will cover 1040 patients. We’re at 800 at the moment and positive to finalize it within the next 10 months. Once we have the results, we’ll file for permanent approval.
A competitor, Newsenselab from Berlin, got its migraine app to the preliminary listing phase but failed to achieve a permanent listing. The company filed for insolvency in August 2022. What do you make of that?
I’m sorry for the team and the founders, who I know. They did a good job, and competition isn’t always bad for business, particularly in a young industry in which many stakeholders still need to be educated on the general topic. Moreover, their approach to treating migraine with a digital migraine diary was quite different from ours. We saw it as a rather complementary approach. I believe it’s a good thing when patients and physicians have the choice between different products which use different treatment angles. We were able to acquire their marketing assets, basically a brand and well-visited website which we want to maintain. They did a fantastic job in educating patients about migraine and we want to continue with this.
It is quite a horror scenario to have the preliminary listing end and get retracted from DiGA, which basically means your sales go to zero. It comes back to the question of how much money and effort you put into the clinical trials, because the more patients you recruit, the better your chances are. The example also shows that it pays to have flexibility in your cost base. We will, for example, work with external sales representatives to ensure this flexibility, and work on new markets and new indications. It’s risky to be a single-product company in healthcare, which is why at Perfood we are building a platform of digital therapeutics where we use our technology stack, our algorithms as well as our evidence and regulatory expertise in a synergetic way.
“Our data show a significant reduction of migraine symptoms after 3 months.”
Before we talk about other indications, let’s stay with your first migraine product for now. How long do patients need to use it?
Our data show a significant reduction of migraine symptoms after 3 months, and a follow-on analysis shows that this is still true 12 months after the treatment. For patients with moderate symptoms, one treatment might therefore already be enough, while other patients might need to follow the treatment for a longer period or repeat it after one or two years.
The treatment costs 690 Euros per quarter. Is there a significant repricing risk stemming from pressure from the health insurers who pay for the treatment?
We built our pricing based on a health economic model. It takes direct and indirect costs for health insurers and society into account and is based on the savings our therapy makes possible. It relies on the efficiency data from our pilot study and will be updated with the data from our confirmatory study. I think our pricing is reasonable if you compare it to generic beta blockers which have side effects that are so severe that 84% of patients are abandoning the therapy within 12 months, or to the treatment with monoclonal antibodies which costs approximately 4,000 Euro per year. All in all, I’d say the risk of repricing is moderate.
Let’s talk about the future then, and the new indications you’re working on. How many will you be able to develop, and in what time frame?
Our goal is to launch several digital therapies all of which are based on our core competencies in technology and nutritional science, for different indications. This will eventually give our company a broad base. A rhythm of around one product launch per year is realistic because we’re working on internationalization at the same time. We’ll go to the US first, then France. We’re currently doing pilot studies for diabetes and will aim for starting the approval procedure in 2023. Other indications where we know that nutrition has decisive effects that we’re looking at are metabolic and inflammatory as well as neurodegenerative diseases, and cancer. We have, together with the German Cancer Research Center in Heidelberg and the University of Lübeck, received a federal grant of 8 million euros to develop a digital therapy for intestinal cancer. With such a project, we’re talking about timeframes of 5 years or more. To internationalize and develop new indications, we will raise further financing from long-term-oriented investors that share our vision.
Written by
WITH US, YOU CANCO-INVEST IN DEEP TECH STARTUPS

Verve's investor network
With annual investments of EUR 60-70 mio, we belong to the top 10% most active startup investors in Europe. We therefore get you into competitive financing rounds alongside other world-class venture capital funds.
We empower you to build your individual portfolio.
More News
28.06.2022
Sympatient is building the first digital anxiety clinic
When startups want to raise money, they can choose between different options. The most commonly used instrument is equity, but other sources of financing such as debt, grants, SAFEs and convertible loans are frequent, too. This article explains what investors need to know about convertible loans, the most common financing form besides equity.
30.11.2020
Perfood (Personalized Diet): EUR 5 million Series A
The German digital therapeutic startup has received 5 million Euro in its Series A funding round. Boehringer Ingelheim Venture Fund led the round.
17.08.2020
“Our core business is the human metabolism”
Eating right is easier than people think, explains nutritional expert Prof. Christian Sina in this interview. But people need to know how different meals affect them, because the reaction is highly individual. Based on this premise, digital health startup MillionFriends was founded.
Startups,Innovation andVenture Capital
Sign up to receive our weekly newsletter and learn about investing in technologies that are changing the world.