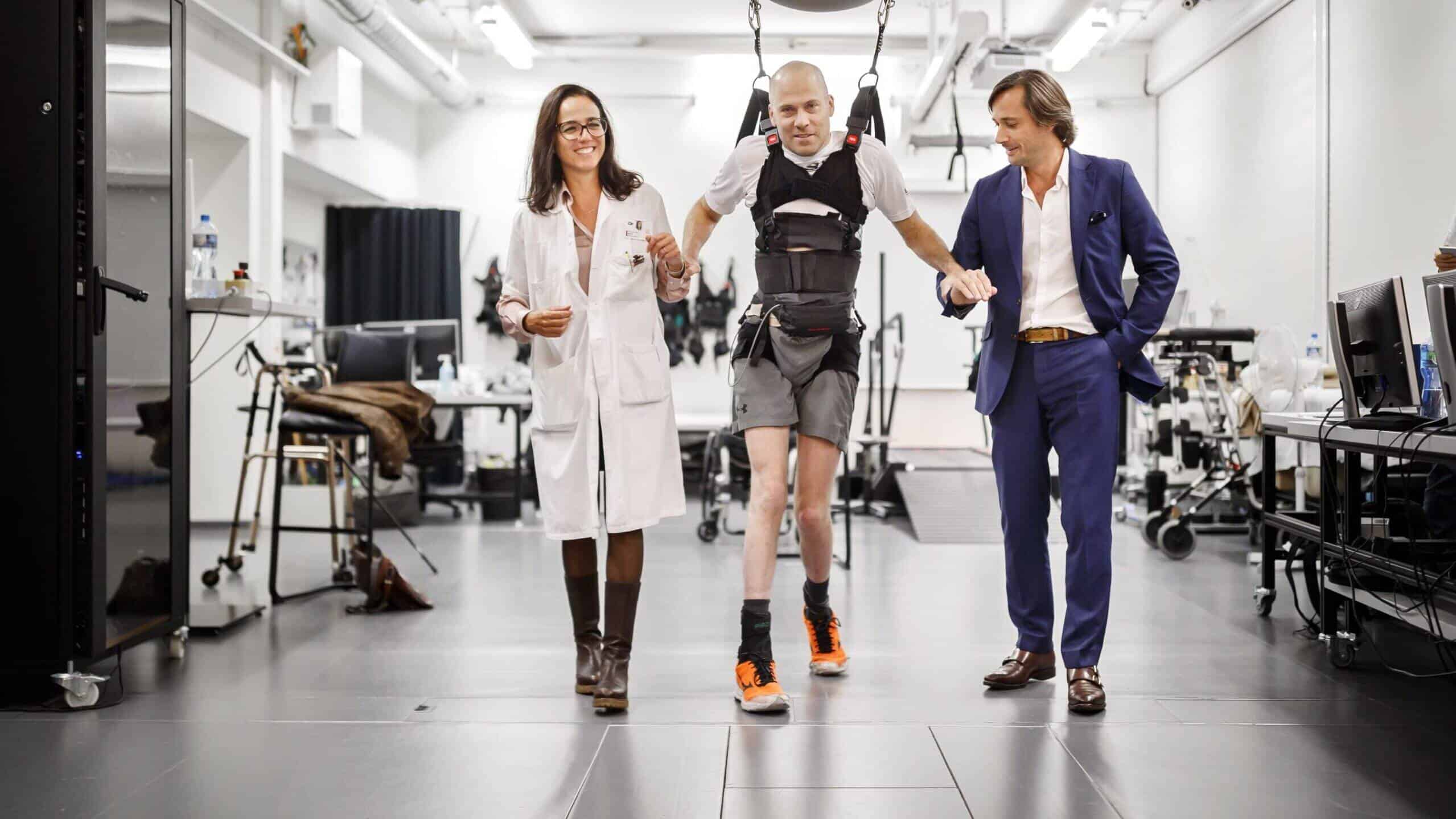
EPFL spin-off ONWARD aims to improve the quality of life of people bound to a wheelchair by spinal cord injury. After the successful IPO in October, CEO Dave Marver looks forward to 2022, when the company is facing important milestones.

CEO, ONWARD
Dave Marver joined ONWARD as CEO in 2020 and took the company public in October 2021 on the Amsterdam and Brussels stock exchanges. ONWARD develops therapies to restore movement, independence, and health in people with spinal cord injury. Verve Ventures invested in ONWARD in spring 2021. Dave has more than 25 years of experience in Medtech, having worked for Medtronic and Cardiac Science, a NASDAQ-listed company where he served as CEO. He was a medtech advisor for the World Bank, a board member of several companies, and a guest lecturer for several universities.
Until recently ONWARD was a private company, backed by deep-pocket investors such as the Onassis family. Why did ONWARD decide to do an IPO so early, when the company still makes substantial losses?
For us, the initial public offering was a way to raise a substantial amount of capital at a fair valuation. To our knowledge, ONWARD did the largest ever European medical device IPO this year and perhaps in history. We raised over USD 100 million to invest in research and development, clinical trials, and the commercialization of our products.
ONWARD is developing two products that can provide health benefits for patients through electric stimulation of the nerves. One device is applied externally, the other is implanted. Before ONWARD can start selling them, you need FDA approval in the US and the CE marking in Europe. How long will that take?
As you described, ONWARD is developing two technology platforms that will enable us to pursue three indications: Restoring strength and function of arms and hands with the external device called ARC-EX and restoring the ability to stand and walk as well as the regulation of blood pressure for the implantable device ARC-IM. The pivotal clinical trial for ARC-EX is ongoing at the moment. We expect to complete the enrolment of patients before the end of Q1 next year. We will then submit our data to the regulator and expect the market authorization and first revenues in 2023. For the implantable device, we expect commercialization to start in late 2024.
“We expect the market authorization and first revenues in 2023.”
Which one is more important for ONWARD?
Ultimately, we expect the implantable device to generate more value. But the external device is an important step to get there, because it will help develop the market by allowing therapists to see the benefit of spinal cord stimulation. This will make them more likely to refer patients to ONWARD later when the implantable device becomes available. There is a role to play for both, it depends on the patients and the use case.
The FDA has granted these products a so-called “Breakthrough Device Designation”. What does this mean?
This designation is a recognition by the FDA that our therapies are truly innovative, address an important unmet medical need, and that there is no alternative currently available. We have received this designation for all the three indications under development. It confers important benefits to us, and compresses the timeline until commercialization. We have more frequent interactions with the FDA, the approval process is streamlined, and it is beneficial for the reimbursement of our therapies. FDA approval is a big challenge for every medtech company, and this designation takes away some hurdles.
What is your go-to-market strategy?
We will be selling to specialized clinics that care for spinal cord injury patients and to hospitals that will implant our device. Fortunately for us, the number of rehabilitation clinics in the EU and US is only about 200, so the number of call points is limited. This means that we can build our own field organization with a very high-touch approach to selling the device.
Will it be hard to gain acceptance from medical practitioners for completely new therapies?
We expect that these therapies will be welcome, since there is no other therapy available that can restore strength and motor function and improve the quality of life. Even for the neurosurgeons that will implant the device there is no training burden, as the procedure is similar to others they are used to.
In the US alone, there are about 300’000 people with spinal cord injury. Such a large market must attract competitors.
ONWARD is a pioneer in spinal cord neurostimulation, and there is very little competition. This has allowed us to amass a large estate of intellectual property with almost 300 pending or granted patents. About half of them we have developed inhouse, and for the other half, we have exclusive licences from leading research institutes. This intellectual property represents a very strong competitive moat.
But investing in a pre-revenue company like ONWARD still means that a lot of things can go wrong until you achieve first sales.
The major risks that medtech companies face have been mitigated to a large extent in ONWARD’s case. The technology risk is largely reduced as the therapies worked well during pilot trials. With the IPO, the funding risk has become insignificant. And our seasoned management team will assure that ongoing clinical trials run smoothly. There is also no reimbursement risk, as we can rely on existing reimbursement codes that cover implantable neuromodulation devices, which are already used today for pain management.

Invest in Startups
As one of Europe’s most active venture capital investors, we grant qualified private investors access to top-tier European startups. With investments starting at EUR/CHF 10’000, you can build your own tailored portfolio over time and diversify across stages and sectors.
You have already led the Nasdaq-listed medtech company Cardiac Science as a CEO. When you look back at ONWARD’s IPO, what was the most important challenge you faced?
I’d say the regulatory and administrative complexity was high. As I explained, we have two different technology platforms, three indications, an extensive R&D pipeline and IP agreements with leading neuroscience institutes in the world. All of this had to be covered in the IPO prospectus, which was around 400 pages long. We listed both in Amsterdam and Brussels because we’re headquartered in the Netherlands and Brussels is Europe’s leading Life Science stock exchange. Dealing with two regulators added complexity to the process. The fundraising itself went smoothly. We have attracted a variety of respected long-only funds from the US, Scandinavia, Europe, and other regions. And we have spoken to quite a few more high-quality funds that are interested to invest as the market capitalization grows. They have certain thresholds below which they cannot invest yet.
“Investors need only to look at the effect our therapies can have on patients.”
As you mentioned ONWARD has two platforms for 3 indications. On one hand, it is reassuring that ONWARD is not a flamingo company resting on just one leg. On the other hand, it makes your equity story more complicated to tell.
If ONWARD were an ordinary company, this might be true. But investors need only to look at the effect our therapies can have on patients. They can watch a video that shows a patient who could not move his toes before being able to stand up and walk again with assistance. When we show these videos, we don’t have to explain much more about why we care about ONWARD’s mission.
ONWARD’s share price has shown some weakness recently. Does this indicate that investors are not in a hurry to invest, since it will take a while before your therapies can be commercialized?
We had to adhere to a quiet period after the IPO which meant that we could not communicate about the progress we have made. And I don’t know if investors are trying to time their entry as late as possible, but as we all know, timing the markets is notoriously difficult. Usually, the markets anticipate good news half a year in advance or so. Personally, I’m focused on the two upcoming major milestones that will create significant value for our shareholders, namely the completion of the clinical trials and the start of the commercialization. We expect the news flow to pick up early next year, when we have finished enrolling patients for the clinical trial.
So much for the imminent future. If you look very far down the road, how much long-term potential is there for ONWARD?
We are developing a completely new market in medtech, and there is reason to believe that our growth profile will be very attractive. We have a very close relationship with Neurorestore, the world’s leading neurotechnology institute led by ONWARD’s co-founder Prof. Grégoire Courtine and Dr. Jocelyne Bloch. And our technology platforms will eventually allow us to add multiple additional indications to those we’re pursuing right now. If you add these elements together, there really is no limit to ONWARD’s growth potential for the next decade.
Written by
WITH US, YOU CANCO-INVEST IN DEEP TECH STARTUPS

Verve's investor network
With annual investments of EUR 60-70 mio, we belong to the top 10% most active startup investors in Europe. We therefore get you into competitive financing rounds alongside other world-class venture capital funds.
We empower you to build your individual portfolio.
More News
01.12.2021
“Astrocast will become the market leader in satellite IoT”
The internet of things is finally taking off, thanks to satellite communication, explains Astrocast’s CFO Kjell Karlsen in this interview. In early 2022, the company will start delivering the communication modules that connect to its satellites.
23.09.2021
“When the stars align, you can’t wait”
In this interview, entrepreneur and investor Gaëtan Marti recounts how difficult it can be to find financing for a startup, how he bootstrapped Atracsys and eventually, despite different plans, sold it to a big MedTech company.
23.03.2021
Professor Courtine and Superman’s Legacy
In 2014 Grégoire Courtine founded the startup Onward to improve the lives of people bound to a wheelchair. The impact of this technology based on neurostimulation seems unreal, almost magical, but will be felt soon.
Startups,Innovation andVenture Capital
Sign up to receive our weekly newsletter and learn about investing in technologies that are changing the world.